Philips introduces new diagnostic device to help prevent childhood pneumonia deaths in low-resource countries
November 12, 2015
Nairobi, Kenya - On the occasion of World Pneumonia Day, Royal Philips (NYSE: PHG, AEX: PHIA) today announced the upcoming release of a Children’s Automated Respiration Monitor, aimed to help improve the diagnosis and treatment of pneumonia in low-resource countries, potentially preventing many of the 935,000 childhood deaths caused by pneumonia each year1. The Children’s Automated Respiration Monitor has the potential to assist community health workers in establishing a more accurate measurement of a sick child’s breathing rate to help improve the diagnosis of pneumonia.
Each year, pneumonia kills more children than AIDS, malaria and tuberculosis combined, and remains the leading infectious cause of death among children under-five, killing nearly 2,5003 children a day, with most victims under two years of age. Every 35 seconds a child dies of pneumonia, with 99 percent of deaths occurring in low-resource settings in developing countries, which are typically rural with poor healthcare facilities, and where treatment is not available for many children4.
One important aspect in diagnosing pneumonia is monitoring a child’s breathing rate. In many emerging markets, community health workers manually count through visual inspection, how many breaths a child takes in the span of one minute. But achieving an accurate count can be difficult, as shallow breaths are hard to detect, children often move around and there may be distractions and other checks to perform.
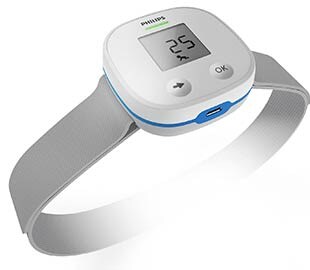
The Philips Children’s Automated Respiration Monitor converts chest movements detected by accelerometers into an accurate breathing count, using specially developed algorithms. The monitor not only provides quantitative feedback, but also qualitative feedback to the healthcare provider based on the World Health Organization’s IMCI 5 (Integrated Management of Childhood Illness) guidelines to diagnose fast breathing rates, which is one of the key vital signs to diagnosing pneumonia.
Accurate diagnosis of breathing counts would support health workers in administering the antibiotics that children with pneumonia need, potentially preventing many of the deaths caused by pneumonia each year. Additionally, accurate diagnosis could help rationalize the use of antibiotics, by potentially reducing unnecessary costs and antibiotics overuse rates, which contributes to the rise of drug-resistant diseases. “The Philips Children’s Automated Respiration Monitor will be a game changer in diagnosing and treating pneumonia,” said Salim Sadruddin, Senior Child Health Advisor at NGO, Save the Children. “If we can remove the subjectivity associated with health workers counting breaths, we can improve the quality of treatment and help improve patient outcomes.” Global child health organizations like UNICEF have made pneumonia a key area of focus in their effort to reduce child mortality in underdeveloped countries throughout the world. UNICEF’s Supply Chain division’s product innovation project called ARIDA6, launched a call for technology to industry in 2011, with the aim to achieve innovation in this space, followed by the publication of a Target Product Profile (TPP)7 for automated respiration monitoring in November of 2014. “As a leading health technology company, Philips’ vision is to improve people’s lives through meaningful innovation”, said JJ van Dongen, CEO Philips Africa. “Today, the population growth is highest in emerging markets like Africa and South East Asia, and innovation can help drive sustainable solutions that bridge the divide between the privileged and lesser privileged sections of society to improve the quality of life at all levels.“ The development of the Philips Children’s Automated Respiration Monitor has been a result of collaboration between the Philips Africa Innovation Hub located in Nairobi, Kenya, the Philips Research team in Eindhoven, The Netherlands and the Philips Innovation Campus in Bangalore, India. Field testing on the Children’s Automated Respiration Monitor was conducted in East Africa and India and improvements in design and technology incorporated on the basis of feedback from local community health workers and clinical officers in these low-resource settings. The Philips Children’s Automated Respiration Monitor is pending CE-marking and is expected to become commercially available from the second quarter of 2016.
1 pending regulatory clearance 2 http://worldpneumoniaday.org/wp-content/uploads/2014/11/Final-WPD-2014-Fact-Sheet1.pdf 3 http://worldpneumoniaday.org/wp-content/uploads/2014/11/Final-WPD-2014-Fact-Sheet1.pdf 4 http://worldpneumoniaday.org/wp-content/uploads/2014/11/Final-WPD-2014-Fact-Sheet1.pdf 6 http://www.unicef.org/innovation/innovation_81722.html
5 http://www.who.int/maternal_child_adolescent/documents/IMCI_chartbooklet/en/
7 http://www.unicef.org/supply/files/Pneumonia_Diagnostics_Aid_Device_TPP_Introduction.pdf
For further information, please contact:
Radhika Choksey
Philips Group Communications - Africa
Tel: +31 62525 9000
Email: radhika.choksey@philips.com
About Royal Philips
Royal Philips (NYSE: PHG, AEX: PHIA) is a diversified health and well-being company, focused on improving people’s lives through meaningful innovation in the areas of Healthcare, Consumer Lifestyle and Lighting. Headquartered in the Netherlands, Philips posted 2014 sales of EUR 21.4 billion and employs approximately 106,000 employees with sales and services in more than 100 countries. The company is a leader in cardiac care, acute care and home healthcare, energy efficient lighting solutions and new lighting applications, as well as male shaving and grooming and oral healthcare. News from Philips is located at www.philips.com/newscenter.
